The results of research on screening for breast cancer with mammography can be presented in ways that make the benefits seem larger or smaller
Similarly, the benefits can be described as avoiding deaths from breast cancer or avoiding deaths from any cause
Part of the debate about the benefits of screening mammography may be related to the differences in the way that the benefits of screening are presented.
The recent recommendations on breast cancer screening released by the Canadian Task Force on Preventive Health Care have created a storm of controversy about the benefits and harms of mammography, particularly for women aged 40 to 49. The recommendations suggest routinely screening women aged 50 to 74 every two to three years with mammography. The recommendations suggest not routinely screening women aged 40 to 49.
There appear to be four broad areas of disagreement about the benefits and harms of mammography:
- how to present the results of the randomized controlled trials of mammography to women, clinicians and policy makers
- how much importance to attach to the results of non-randomized studies
- whether the early detection of a breast cancer in a woman means that mammography saved her life
- how to balance the benefits and risks of mammography when making a decision about whether or not to undergo mammography.
We will be posting a series of brief articles about each of these topics between now and the winter holidays. This article focuses on the evidence for screening women between the ages of 40 to 49.
Differences between the Canadian Task Force on Preventive Health Care and the Canadian Breast Cancer Foundation
The Canadian Task Force on Preventive Health Care (Task Force) notes that slightly more than 2000 women “would need to be screened … about once every two years over a median of about 11 years to prevent a single death from breast cancer.
A press release from the Canadian Breast Cancer Foundation (CBCF) responding to these recommendations quotes their CEO, Sandra Palmaro, as saying “scientific evidence demonstrates that earlier detection and diagnosis can save lives among women 40-49 by at least 25%.”
Are the statements from these two organizations compatible?
There appears to be two areas of disagreement. The first is about the magnitude of the benefit of screening – 2018 women needing to be screened to prevent one breast cancer death according to the Task Force, versus a 25% increase in lives saved according to the CBCF. The second is about how the benefit of screening is measured – a single death from breast cancer prevented from the Task Force, versus lives saved from the CBCF.
The difference between the relative and absolute benefits of screening
The estimates of the magnitude of the benefit from screening used by these two organizations may not be as far apart as it first appears. The Task Force based its estimate on the benefit of screening from 8 studies involving almost 350,000 women. Study participants were randomized to either undergo screening mammography or not. These studies found that the risk of death from breast cancer was decreased by 15%, which isn’t that much different from the 25% risk reduction quoted by the CBCF. It is important to clarify where the estimates of benefit come from.
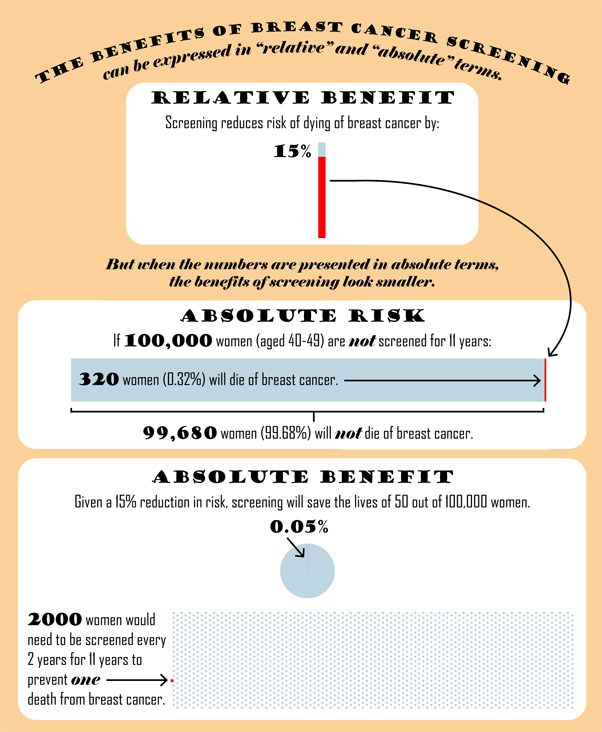
The difference is that the Task Force presented its figures as absolute risk reductions, while the CBCF presented its figures as relative risk reductions.
The infographic below uses the Task Force data to explain the difference between describing the benefits of mammography in relative and absolute terms.
What is an absolute risk reduction?
After 11 years of screening, 0.32% (or 1 in 313) of women in the randomized trials who were not screened, had died of breast cancer. If the risk of dying from breast cancer is decreased by 15% with screening (which is what the Task Force suggests), this means that 0.27% (or 1 in 370) of women who are screened for 11 years would die from breast cancer. The absolute difference between these two numbers is just 0.05%. Another way of saying this is that about 2000 women will need to be screened to prevent one death from breast cancer, 11 years after beginning regular screening.
The CBCF states that the relative benefit from screening is actually 25% rather than the15% that the Task Force suggested. This would mean that 0.24% (or 1 in 417) of women who are screened would die from breast cancer, which would require that 1250 women need to be screened to prevent one death from breast cancer (rather than the 2100 estimated by the Task Force). In either scenario, well over 1000 women need to be screened to prevent one death from breast cancer.
When talking about the benefits of any medical test or treatment in health care, it is important that people are informed about the absolute benefits. Some may argue that a focus on absolute benefits is biased against preventive interventions because the absolute benefits of prevention are usually small. For example, the absolute benefits of wearing a seat belt while driving are likely smaller than the absolute benefits of mammographic screening, yet it is illegal not to wear a seatbelt. However wearing a seatbelt causes virtually no harm (we will address the potential harms of mammography in a subsequent article) and the cost of installing seatbelts in cars and wearing a seatbelt is small.
Deaths from breast cancer versus death from any cause
One wouldn’t expect a screening programme for breast cancer to decrease death from any cause other than breast cancer. Therefore, it makes sense to present the benefits of a mammography screening program in terms of the number of deaths from breast cancer prevented.
At the same time, women contemplating mammography screening for breast cancer may be interested in knowing whether having a mammogram can decrease their risk of dying from all causes.
The Task Force described the benefits of mammography in terms of deaths from breast cancer. However, this doesn’t provide any information about the impact of screening on women’s risk of death from any cause. Breast cancer is a common cancer in women, and most women aged 40 to 49 are not yet at a high risk of heart disease and other “competing” causes of death. Therefore, one might think that their risk of death from any cause would be decreased by mammography. However, that is not the case – the Task Force review notes that there was no difference in the likelihood of death in women who were screened and those who were not screened in the 350,000 women in the randomized trails of mammographic screening in women 40-49 years of age.
The CBCF press release mentions deaths from breast cancer and deaths from any cause interchangeably, even though they are different. When presenting the benefits of screening programs to women, it is important to be very clear about whether one is talking about deaths from breast cancer or deaths from any cause.


The comments section is closed.
We agree with Dr. Yaffe that reporting both absolute and relative benefits of screening is important – which is why we provided both in our guideline and the accompanying systematic review. We also agree that years of life saved by screening might be a useful metric for policy-makers. Unfortunately, these data were not reported in the randomized trials of screening mammography. More importantly, these data are unlikely to influence the decision of an individual woman about screening: 40 year old women are well aware that (on average) they have many more years of life remaining than does a 70 year old woman.
Focusing on lives saved (in both relative and absolute terms) and leaving the decision to individual women appears more appropriate than the simplistic suggestion that all women should be screened simply on the basis of their age. Is our approach really “scientifically unsupportable”? Or does this term just mean that Dr. Yaffe does not agree with involving women in decision-making, and would prefer a more paternalistic approach?
Dr. Yaffe rejects our estimates of benefit (based on a systematic review of the literature) in favor of his own back-of-the-envelope calculations – and discards 7 of 8 published trials in favor of the one that best supports his views. Although it is possible that newer technology has increased the benefit of screening, it is also possible that it has actually had no effect – or has increased the likelihood of harm by detecting more subtle abnormalities that are not actually cancer. The speculation that screening improves quality of life through earlier detection (permitting less aggressive treatment) is unfounded: the reductions in quality of life associated by overdiagnosis and false positives appear far more relevant clinically.
Our guideline is intended to help individual women to make an informed decision about the potential harms and benefits of screening – and we agree with the Editors of healthydebate.ca that decision aids (like the infographic above) are a useful tool to facilitate discussions between doctors and patients.
It will be difficult to determine the cost-effectiveness of screening mammography with precision. It will be even more difficult to determine the incremental cost effectiveness of screening programs.
There is a classic paper in Risk Analysis (http://bit.ly/tX6BzR) that looked at the cost-effectiveness of 500 different interventions. Some of the various seat belt interventions were very cost effective. So were some of the mammography interventions. For example, one-time screening at age 50 of 60 is almost certainly good value for money. The incremental benefit of more frequent screening is of course smaller. Deciding where exactly the diminishing returns become so small as to be insignificant is an impossible task, so it is no surprise that the CTFPHC is being criticized from both sides.
Over the past week I have written extensively that the recommendations of the Canadian Task Force on Preventive Health Care (CTFPHC) are scientifically unsupportable. Your current article focuses on statement of the benefits, so I will reserve my comments on “harms” until your next article. I have several concerns about the points made in this article. You state that there is not much difference between 15% and 25% mortality reduction. This is almost a factor of two, an appreciable benefit in any manoeuvre. You correctly state that the risk of death in a single woman over a small range of time is small. The risk accumulates over several decades of life to result in breast cancer being the second largest cause of cancer death in women. When applied to a small absolute risk of death, this difference is small, but applied to the Canadian population of women in their 40s it would result in the difference of estimating the number of lives saved as 120 instead of the 200 that would result from the use of data based on modern mammography, rather than obsolete 1980s imaging. Most lay people would have a better sense of what this means than when presented with the small numbers 0.27% versus 0.32% of women dying. So we should begin by using data from the best trials that used relevant technology to estimate benefit. This disqualifies seven of the 8 randomized trials that the CTFPHC used because those studies used mammography that would not even be remotely acceptable by today’s standards. The remaining trial (performed in the UK) showed a 24% mortality reduction (in the women who actually were screened as opposed to simply being allocated to the screening arm of the trial) and this was backed up by observational trials of screening actually delivered to the public in the real world where a mortality reduction no less than 26% was seen.
Secondly, the CTFPHC specified the benefit of lives saved and ignored the benefit in terms of years of life saved that was calculated by the US Evidence Review Group tha informed the US Task Force for its 2009 report. In this way they ignored the benefit of saving the lives of the younger women who would lose more years of life if they died prematurely due to breast cancer. If the goal is to convey the benefits attributable to screening in a clear and meaningful way, certainly this aspect of benefit should have been conveyed. Finally the benefits in terms of reduced morbidity that often come from treatment of less advanced disease found through screening were ignored in the Recommendations. This quality of life issue is one that is likely to be important to women when considering whether to be screened or not.
The statement of the number of women required to screen to save a life is also incorrect. Here is a simple, but apt calculation: For 1000 women in their 40s, screened annually for 10 years, 20 cancers would be expected to be found. About 25% or 5 of these women would die in the absence of screening and about 25% of these deaths or 1.25 (please forgive the fractions!) would be saved. So the number of women screened for 10 years to save a life would be 1000/1.25 = 800. This agrees well with the estimate of 746 presented this week at the Radiological Society of North America conference (Helvie, M and Hendrick RE).
Finally, a reference is made to seat belts. We are in the midst of a study of the cost-effectiveness of mammography screening and will report on this next year, but I am quite confident that the cost per year of life saved will be considerably less for screening than for seat belts.
I suggest that both absolute and relative terms be used in communicating benefits and harms, but that a much more complete description be presented. This should include an estimate of the reductions in the years of life lost and conditions related to earlier detection under which chemotherapy may not be necessary.
Martin J. Yaffe PhD
Sunnybrook Research Institute
University of Toronto
Director, Smarter Imaging Program, Ontario Institute for Cancer Research
Risk reduction, whether described in relative or absolute terms, is difficult to understand for many, let alone explain. Most reasonable people would conclude that a “risk increase of 25%, or even 15%”, would be unacceptable, even when avoiding that risk is accompanied by a litany of false-positive ramifications. So we need to do a better job of explaining this in context – if deaths rise from 1 to 2 out of ten, that means one thing; if from 1 to 2 in a million, quite another.
Beyond the confusion lies the question of how we allocate scarce public health care dollars to derive the optimal mix of health outcomes – a debate over which some jurisdictions have had greater success than others, like ours, where we consider resources to be endless (despite borrowing billions each year to pay the bills). We need a discussion on the relative merits of home care for the elderly vs mental health access and services for teens vs critical care in hopeless situations vs cancer screening in the 40-50 age cohort, etc etc.
It would also be useful to have comparative “number needed to screen” values presented to provide further context of the values of 1250 and 2100 mentioned in the article. For example, how many women over the age of 50 years need to be screened to avoid a death due to breast cancer in that group? I imagine it is smaller, but how much smaller? Also, how do these numbers compare to other treatment interventions such as taking a statin to avoid a myocardial infarction? I recall that several years ago an article with a list of NNTs was published in a Heath Technology Assessment journal. Perhaps it could be located and summarized. Thanks!
Thanks for the comment. To answer your first question, the Canadian Task Force on Preventive Care notes that 720 women between the ages of 50 to 69 need to be screened every 2-3 years over an 11 year period of time to prevent 1 death from breast cancer. The Task Force also says that this would results in about 204 women having a false-positive result on a mammogram, and 26 having an unnecessary biopsy of their breast.
You can access more information around the guidelines here: http://www.canadiantaskforce.ca/recommendations/2011_01_eng.html
To respond to your second question, here’s a useful resource that you may want to look at that has the NNT across conditions with subsets of medical disciplines: http://ktclearinghouse.ca/cebm/glossary/nnt , though if anyone else has the reference to the paper BJ refers to, please share it in the comments section or email info@healthydebate.ca
Thanks.