In 2003, the sequence of the human genome – our genetic code – was revealed. For the first time, we now had a complete list of human genes (about 20,000), and could realistically start to address what these genes did, how they make us human, how the body works and what happens when things go wrong. It truly was a momentous event in human history. They were exciting times.
In 2013, on the occasion of the 10th anniversary of the sequencing of the human genome, a group of us was asked to write a Comment for Nature magazine on how the biomedical research community responded to this opportunity. And so we examined the amount of research on each gene before the genome was sequenced and ten years after. We wondered if the availability of our genome sequence had made a transformative impact on how we did biomedical research. Had biomedical researchers rushed to study the newly discovered genes and find out how they might affect human biology and disease? Was there a gold rush for the frontier?
What we found was disheartening. By counting the number of publications on each gene or gene product, we found that more than 20% of all human genes still have fewer publications than fingers on my hand. There was no gold rush. Rather, scientists appear to be quite happy to remain in their comfort zones – the genes that had the most publications in 2003 remained the “hottest” in 2012, with few exceptions (those that had since become the targets of drugs).
Why the reluctance to be pioneers? Some in the scientific community rationalized that the genes/proteins we were studying in 1999 were most “important”. This face-saving argument was not supported by the evidence. Genetic studies now implicate many of the “unstudied” genes in disease. It is hard not to conclude that we still study those genes mostly because we have always studied them.
But our analysis in 2013 reported global trends. Within this larger view of the biomedical ecosystem, how does Canadian science map? Are we more daring? Does our system encourage risk? Are we the pioneers?
Unfortunately, the evidence does not suggest this either.
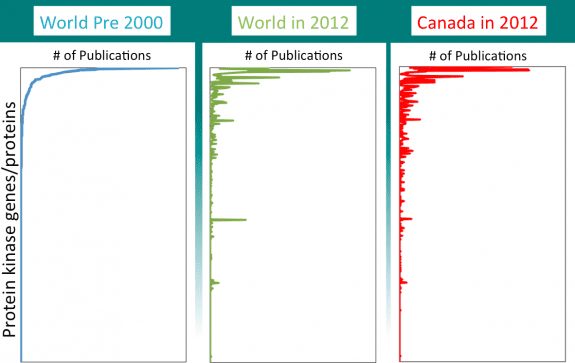
Using a similar methodology, we mapped “publications in 2014 per gene” for all papers on a family of genes/proteins known as protein kinases. These are incredibly interesting enzymes, critical both for regulating cell activity and as targets for medicines. Herceptin and Gleevec, two of the most revolutionary cancer drugs, each target a protein kinase.
In the Figure are three graphs. In each we plotted numbers of publications (across the top) on each of the ~500 individual protein kinases genes/proteins (on the side). In all graphs, the kinases were ranked top to bottom according to their popularity (number of publications) in the year 2000. On the left is shown the world’s total output of publications prior to 2000 (about when the first draft of the human genome sequence was published). In the middle is shown the kinases, ordered in the same “pre-genome” ranking, but counting the world’s total research publications per kinase in 2014. And on the right are publications only from Canadian scientists in 2014. As might be expected, the absolute numbers of publications are different in each of the three graphs, so we normalized to make an easier comparison.
There are three clear observations.
- Most of the world’s research publications on protein kinases are focused on a small number of them.
- Most of the kinases that are currently “hot” are the same ones that were “hot” more than 15 years ago. The exceptions are targets for new medicines (B-raf) and kinase genes whose mutation predisposes for a disease (such as Parkinson’s)
- The pattern of research activity in Canada resembles the world’s activity in an uncanny (and distressing) way
And there are three unsettling take-home messages.
- The “ecosystem” that supports biomedical research is geared toward supporting productive science, but not necessarily innovative science. Although scientists are ostensibly trained to be risk takers, empirically, they are not. It may be that the ecosystem in which we work, from promotion, to peer recognition, securing grants and getting papers accepted in journals, makes taking risks difficult.
- Canadians do not preferentially carry out science at the frontier. The publication pattern of Canadian science is eerily similar to that of the world’s science (and indeed to that of any individual country). We’re part of the pack. Why does this happen? It’s likely because science is global but research funding is parochial. Part of the power of globalization is that all scientists in the world have access to the same scientific literature. But consequently, many scientists have very similar ideas on what to do next. And each can seek funding for these experiments from their national (or provincial) governments. And because there are smart scientists everywhere, they will get funding. And it follows that the same idea can get funded dozens or more different times. And while this encourages healthy competition as well as provides checks and balances to ensure that scientific discoveries can be replicated, it also encourages massive, and likely wasteful, duplication of effort.
- Canadian scientists are having too little impact. Canada has among the world’s most competitive scientists, by any metric. But if our publication patterns mirror those of every other country, is our aggregate research effort mostly redundant? If we stopped all Canadian science, would it make any difference to biomedicine?
These are troubling, but important issues because, despite massive amounts of research funding globally, we are making too little progress in the prevention, diagnosis and treatment of diseases.
I submit that this provides an enormous opportunity for Canadian biomedical research to have a great impact on global science. It should be evident that research on the “hot” areas will continue even if all Canadian biomedical research stops. So why not leave the currently “hot” genes to the rest of the world and adopt a policy to support Canadian pioneers?
If Canada encourages and supports these pioneers, everyone in Canada and around the world wins. If the cures reside within the “hot” genes, then someone, somewhere, will still find them. If the cures reside within the greater unknown, then it will be Canadians that discover them and the world will gain as a result.
The next Klondike is out there, but only to those who dare to venture forth.


The comments section is closed.
I think that you understand some of the limitations. It’s easier to build arguments that something of value will come of the research if there is already literature to make that case. We also have the issue that there are no sources for pilot project funding in much of the country and grants aren’t funded without data. That leaves only well funded laboratories to explore that space. Finally, we have a federal government AND many charities emphasizing the application of science. Perhaps you have a suggestion as to how we might get beyond those barriers to achieve what you suggest we should be doing?
I don’t think anything will/can change until we change the peer-review process, which cannot happen from within
Perhaps set aside a significant amount of funding for a DARPA-like program in which a few folks make the call? (DARPA is a department of defense program in which a few project managers control a sizable amount of money to fund exciting but potentially risky ideas (http://en.wikipedia.org/wiki/DARPA))