Update: As of May 10, the National Advisory Committee on Immunization (NACI) recommends that the AstraZeneca COVID-19 vaccine should not be used in adults under 30 years of age due to a rare but serious side effect of blood clotting.
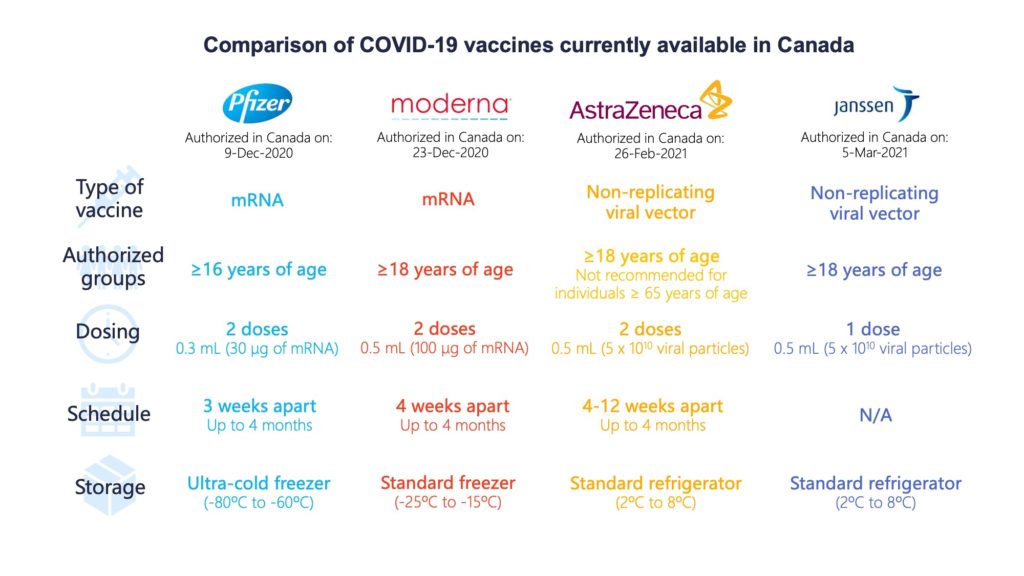
As more and more Canadians become eligible for vaccination in what has become a crowded landscape of COVID-19 vaccines, many may wonder what to expect when they roll up their sleeves. There are now four vaccines available to Canadians. AstraZeneca and the University of Oxford’s vaccine as well as the first single-dose shot from Janssen, a unit of Johnson & Johnson, were recently authorized by Health Canada, joining the Pfizer-BioNTech and Moderna mRNA vaccines approved in December.
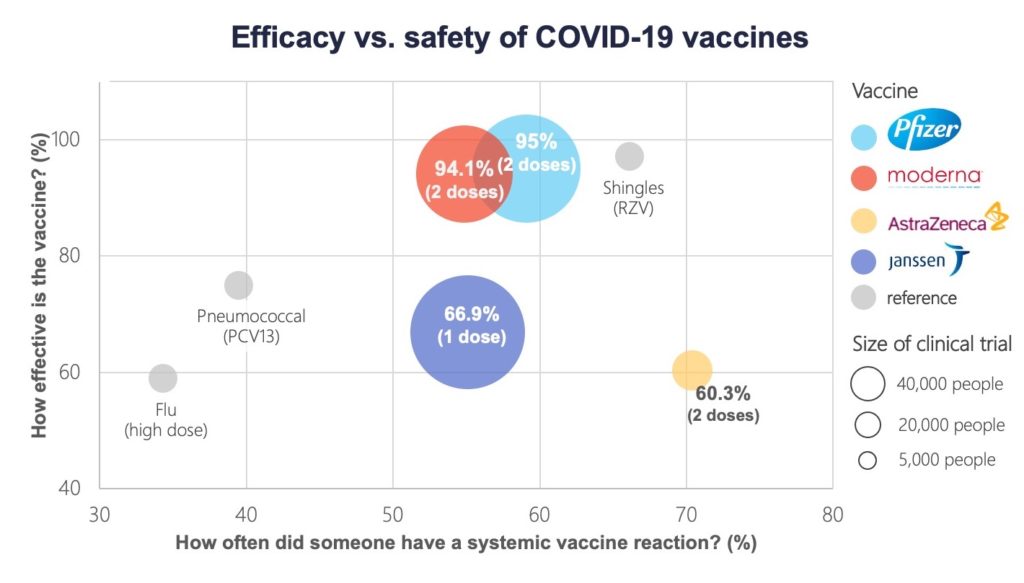
How do the four vaccines compare in efficacy and safety?
The Pfizer and Moderna vaccines have the highest efficacy at around 95 per cent. Lower efficacy, in the 60 to 70 per cent range, was found for the AstraZeneca and Janssen products. (Efficacy measures how many vaccinated people contract COVID-19 compared with how many infections occur in the placebo or control group. Put another way, it represents the proportion of COVID-19 infections that could be prevented by vaccination).
Side effects are common in all four. They appear to be much the same as some vaccines routinely recommended for older adults like the shingles vaccine, but worse than other vaccines like the high-dose flu shot. In the AstraZeneca trial, in which the control group received a meningococcal vaccine rather than a placebo, a similar proportion of younger participants experienced reactions to the meningococcal vaccine and the COVID-19 vaccine.
Older adults appear to have fewer reactions that affect the whole body, such as fevers, while enjoying similar or even higher protection than younger adults.
However, we should be cautious in comparing these trials since they used different ways to measure efficacy, says Alison Thompson, an associate professor who studies public health policy and ethics at the University of Toronto.
“I think the Johnson and Johnson (Janssen) one had much better clinical endpoints in the trials … there was a pretty low bar for establishing efficacy in the other trials,” she says.
The Pfizer and Moderna trials looked at prevention of any lab-confirmed infection starting at least seven to 14 days after the last vaccine dose in those who had symptoms. However, the Janssen trial set a higher bar, looking to prevent moderate to severe or critical infection. “As we get more data about the kind of efficacy that they have over the longer term, we may see those (efficacy) numbers come down significantly for the mRNA (Pfizer and Moderna) vaccines,” says Thompson.
The lower efficacy in the AstraZeneca and Janssen trials may also be explained by a greater number of infections from variants of concern, which emerged after the Pfizer and Moderna trials were completed and against which vaccine protection is expected to be lower. In the Janssen trial, for example, efficacy was 74 per cent in the United States but dropped to 52 per cent in South Africa, where almost 95 per cent of cases were due to the B.1.351 variant. At least 136 cases of this variant had been detected in Canada as of March 8.
We should be cautious in comparing these trials since they used different ways to measure efficacy, says Alison Thompson, associate professor at University of Toronto.
Comparisons should also take into account differences in the number and timing of doses. However, the National Advisory Committee on Immunization expects that short-term efficacy will likely remain high, even with an extended four-month interval between doses.
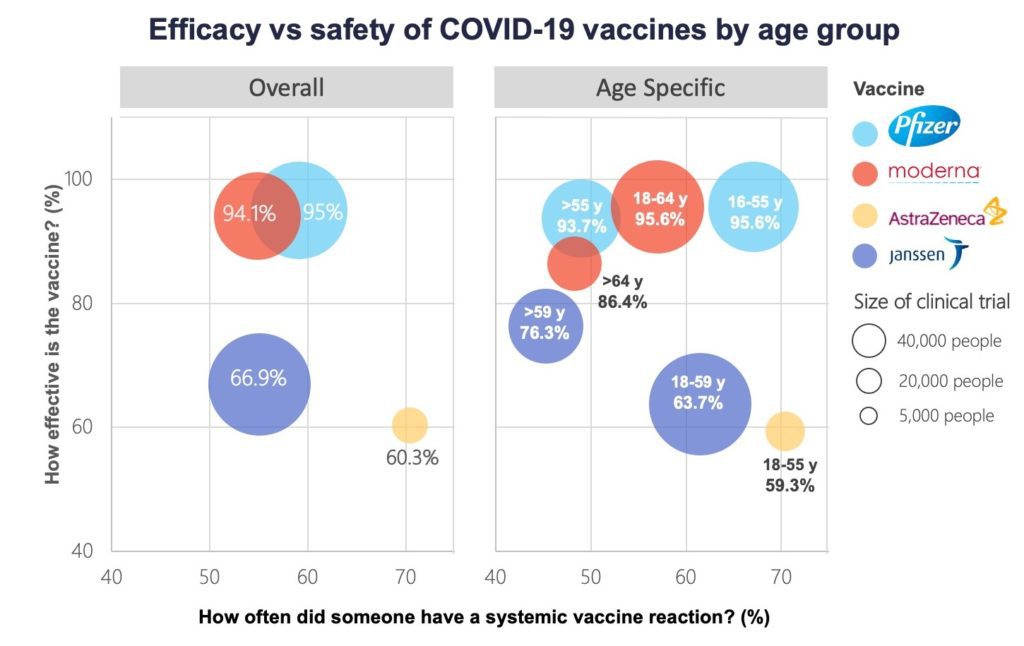
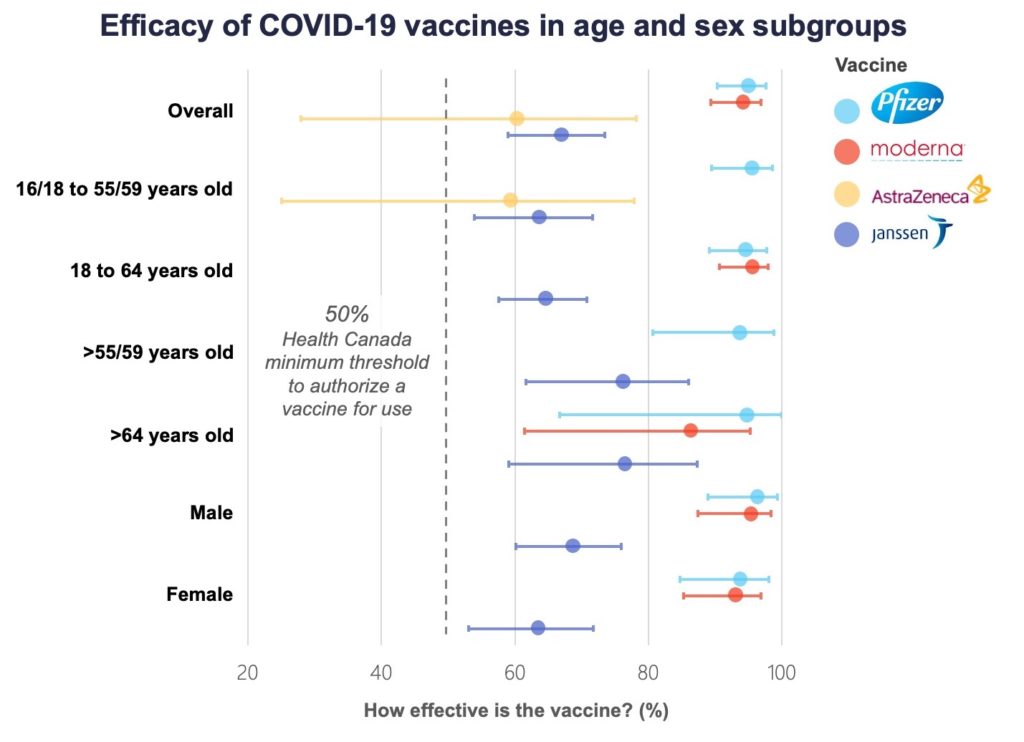
For AstraZeneca-Oxford vaccine data not shown for older adults >55 years due to a limited number of participants in this age group.1,2,3,4
How well do these vaccines work in different populations?
The vaccines appear to work equally well across subgroups defined by age, sex, race, ethnicity and among those at risk for developing severe COVID-19. All four seem to protect against more severe cases and hospitalization, but evidence is still limited.
Importantly, all four vaccines exceed Health Canada’s 50 per cent efficacy standard. “While each of the vaccines that Health Canada has authorized has different efficacy numbers, the reality is that you will have a greatly reduced chance of getting COVID-19 with any of the vaccines that have been authorized,” Supriya Sharma, Health Canada’s chief medical adviser, said in a press conference on March 5. “I would not hesitate to roll up my sleeve to get any of (them).”
The National Advisory Committee on Immunization advised in early March against using the AstraZeneca shot in adults aged 65 years and older, citing a lack of efficacy data for that age group. As more data comes in, “We’ll be able to make better decisions about who ought to get which vaccines,” Thompson predicts.
What are the expected side effects?
Just over half of recipients in the clinical trials for all four vaccines experienced at least one reaction affecting their entire body, like fatigue or muscle aches, after their first dose; this increased to 60 to 80 per cent after the second dose.
Side effects were more common after the second shot of the Pfizer and Moderna mRNA vaccines, while AstraZeneca recipients reported them more frequently after the first dose.
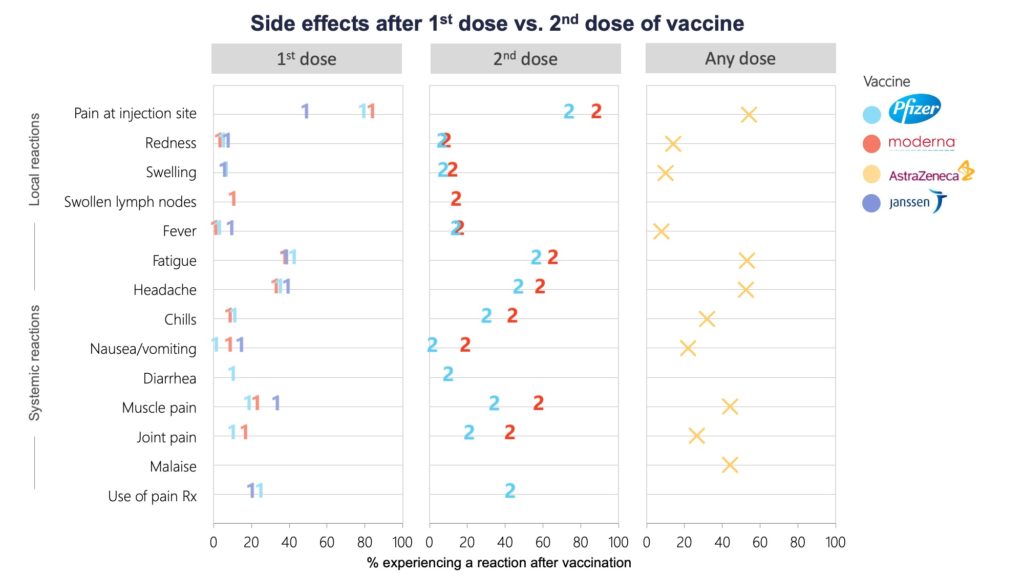
Rx=prescription medication. Per cent of participants in vaccine group experiencing a solicited local or systemic reaction within 7 days following the 1st or 2nd dose.5
Most symptoms were mild to moderate and disappeared after a few days. Severe reactions – defined as those that prevent daily activities or require medical attention – were rare among all trial participants. Most of the side effects are typical for any inoculation. “A little bit of myalgia, fatigue, headache and then injection site reactions, like localized redness, tenderness or swelling are common with all vaccines,” says Lacey Robinson, an allergist and immunologist at Massachusetts General Hospital in Boston.
“Our advice to Canadians is to get whichever vaccine is available to you,” says Supriya Sharma, Health Canada’s chief medical adviser.
Nonetheless, some frontline health-care providers, who were first in line to receive COVID-19 vaccines, say their reactions were enough to miss a day of work, particularly after the second shot of the Pfizer or Moderna vaccines. “I had mine last night and I’m melting into the couch,” said Yuliya Rackal, a family doctor in Toronto, after getting her first Pfizer shot.
For others, however, the reaction has been much milder. “Really painless,” said Karen Swirsky, a family doctor at St. Michael’s Hospital in Toronto, though she adds that she took pain medication for a sore arm after her second Pfizer jab. “Very similar to what I would get with a seasonal flu shot,” said Marvin Gelkopf, another Toronto-area family physician, who described his experience of the Shingrix (shingles) vaccine as “way worse than this.”
Side effects are also more common in younger adults, who make up the bulk of essential workers.
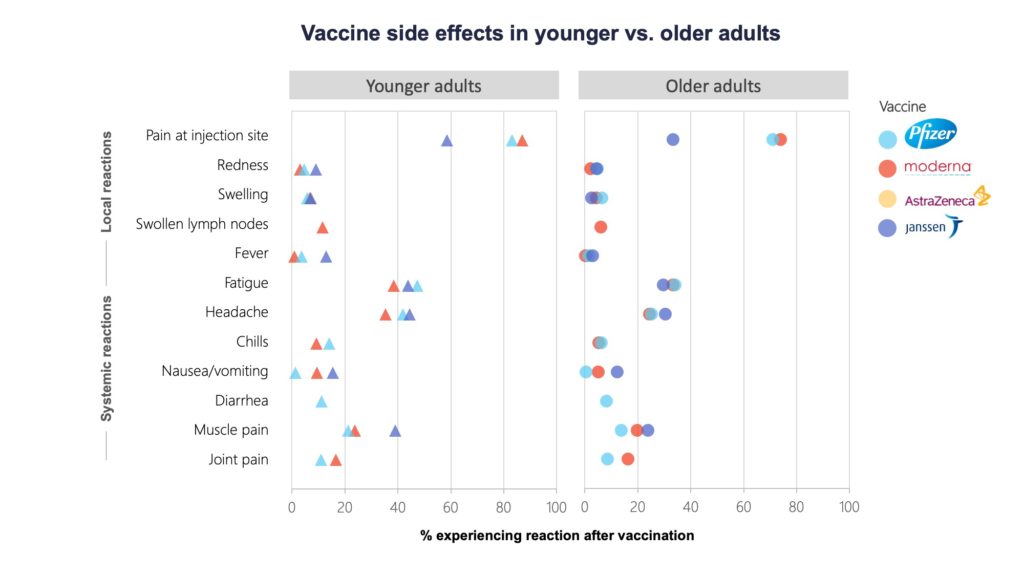
Per cent of participants in vaccine group experiencing a solicited local or systemic reaction within 7 days following the 1st dose. In Pfizer-BioNTech trial, younger adults were those 18-55 years of age and older adults were those >55 years of age. In Moderna trial, younger adults were those 18-64 years of age and older adults were those >64 years of age. In Janssen trial, younger adults were those 18-59 years of age and older adults were those >59 years of age. 5
The frequency and type of reaction may depend on which vaccine you receive. “We certainly are seeing more delayed injection site reactions with the Moderna vaccine, which was also seen in the trials, more commonly about a week after vaccination,” says Robinson. However, she adds, “it probably is a great sign that you’re mounting a really good immune response to the vaccine.”
For those who do experience side effects, physicians recommend at-home treatment like Tylenol or Motrin for pain relief or ice on the arm. Generally the reactions will go away after a few days. “Most people are feeling back to normal very quickly,” says Robinson.
Allergic reactions like anaphylaxis are rare but treatable complications. They occur most often in people with a history of allergic reactions.
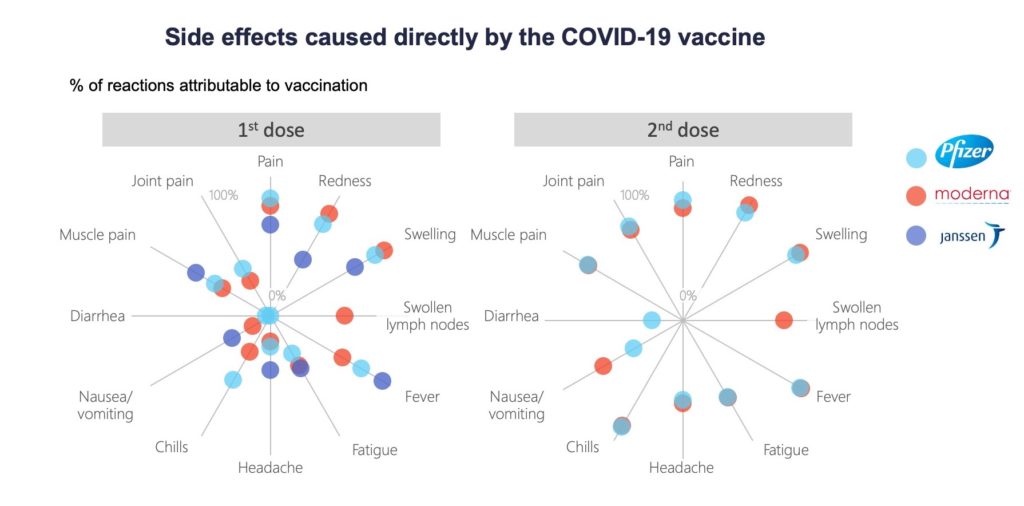
Percent of solicited local or systemic reactions within 7 days attributed to vaccination among vaccinated participants calculated as the percent in vaccine group minus percent in placebo group divided by percent in vaccine group.5
Other differences
The four vaccines use slightly different ways to trick the immune system into producing a response. The Pfizer and Moderna vaccines use messenger RNA, or mRNA, to deliver genetic instructions to the host’s immune cells to build copies of the viral spike protein. AstraZeneca and Janssen hijack another respiratory virus, known as adenovirus, to deliver these instructions.
Both the AstraZeneca and Janssen products can be stored in refrigerators while the Pfizer and Moderna mRNA vaccines, which are less stable at those temperatures, must be stored in freezers. This has already raised issues of equitable access. Because of extreme temperature storage requirements, the Pfizer and Moderna vaccines can only be administered in settings with the necessary freezers, like hospital clinics in major urban centres.
The bottom line is that most Canadians will not have a choice of vaccines due to limited supply.
“Our advice to Canadians is to get whichever vaccine is available to you. The longer you wait to get vaccinated, the longer the time goes by that you are not protected,” says Sharma.
As of March 8, Canada had administered a total of 2.5 million doses. Across Canada, almost two million people have gotten at least one dose and more than 579,000 people, or about 2 per cent of the adult population, are fully vaccinated. Canada has signed agreements for up to 180 million doses in total with manufacturers of the four authorized vaccines: up to 76 million doses for Pfizer, 44 million for Moderna, 22 million for AstraZeneca and up to 38 million for Janssen.
Disclaimer:
Differences in study population, eligibility criteria and study protocols should be considered when comparing data across clinical trials.
Graphics Footnotes:
- Vaccine efficacy measured against primary endpoint of symptomatic COVID-19 infection confirmed by RT-PCR starting at least 7-14 days after last vaccine dose among participants without evidence of prior infection.
- For Janssen vaccine, co-primary endpoints were moderate to severe/critical COVID-19 confirmed by central laboratory occurring at least 14 days after vaccination among participants without evidence of prior infection.
- For AstraZeneca-Oxford vaccine, only the standard dose/standard dose regime for the UK study shown.
- Reactiveness calculated as the per cent of participants in vaccine group experiencing a solicited systemic reaction within 7 days after the 1st dose.
- For nausea/vomiting, only vomiting assessed in Pfizer-BioNTech trial and only nausea assessed in Janssen trial.
Data Sources:
- Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2020 Dec 30. doi: 10.1056/NEJMoa2035389.
- Janssen Biotech, Inc. Vaccines and Related Biological Products Advisory Committee Meeting February 26, 2021. FDA Briefing Document. Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. U.S. Food and Drug Administration; 2021. Available from: https://www.fda.gov/media/146217/download (accessed 2021 Feb 24).
- Medicines and Healthcare Produces Regulatory Agency. Public Assessment Report Authorisation for Temporary Supply. COVID-19 Vaccine AstraZeneca, solution for injection in multidose container COVID-19 Vaccine (ChAdOx1-S [recombinant]). London: Government of United Kingdom; 2020. Available from: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca.
- Moderna. Vaccines and Related Biological Products Advisory Committee Meeting December 17, 2020. FDA Briefing Document. Moderna COVID-19 Vaccine. U.S. Food and Drug Administration; 2020. Available from: https://www.fda.gov/media/144434/download (accessed 2020 Dec 15).
- Pfizer and BioNTech. Vaccines and Related Biological Products Advisory Committee Meeting December 10, 2020. FDA Briefing Document. Pfizer-BioNTech COVID-19 Vaccine. U.S. Food and Drug Administration; 2020. Available from: https://www.fda.gov/media/144245/download (accessed 2020 Dec 15).
- Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020 Dec 10. doi: 10.1056/NEJMoa2034577.
- Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979-93.
- Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111.


The comments section is closed.
https://healthydebate.ca/2021/03/topic/comparing-vaccines/ says AZ not recommended for over 65s due to low efficacy with this group. Australia does not recommend AZ for under 60s due to risk of adverse reactions in this group. Doesn’t leave a lot of room for using AZ does it?
Very clear and informative. Thank you!
WOWWWW THANK YOU! Very clear, easy to understand, and thorough. Finally, something that feels like a more objective report on the data, not just regurgitation of the propaganda for Big Pharma.
I thought u were gonna make 1, 2, 3 and 4. And I thought there was a jj vaccine too?
Can I drink alcoholic drinks after getting the vaccine?
yes
AstraZeneca side effects are not listed in the side effect charts. Why is this? My other comment is that I agree with Stephen Quan that letters should be used to represent vaccines (M, S, A) rather than shapes to display the information on the charts.
Thank you for the very good charts. I have a suggestion however. Would you be able to use colored letters instead of numbers or shapes? For example: a “P” for Pfizer, an “M” for Moderna, etc.
What about “protection” against new variant?
Time to do a new article examining the efficacy of one dose versus two. Should be easy to get stats on this, since millions have had one shot and are now waiting months before getting second shot. I think I read that one manufacturer was stating their first dose was providing 90% protection. People need confirmation of the level of protection the have during the months-long period between shots. Duration of protection should also be discussed in more detail. We now have up to 4 months of vaccine history to give credence to stated protection levels
Can you please tell me your thoughts on what vaccine would be best c9verd for being safe fr9m virus when on an immune suppressant…Imuran to be exact..Thank you..
Also what % of coverage will my body develop since immune is down..Thanks
Just want to say Thank you for the research and putting out such an easy read informative info.
I am greatly appreciative ! Much Respect
Why you can’t compare Covid-19 vaccines: https://youtu.be/K3odScka55A
More questions: April 19th, 2020.
What percent of vaccines given are placebos? If you have a placebo then you are not really vaccinated. Are they still giving placebos and if so, do people getting the vaccines know this?
All the vaccines given to the public are the vaccines stated on the label. Placebos were only used during the phase 3 trials so that the effectiveness can be calculated. If you’ve been given a “vaccination”, then you can be sure you’ve been given the real thing. I understand that one afternoon in York Region a few people were given the saline (salt) solution used to dilute the vaccine by mistake. The error was found the same day, and the people were contacted to return and receive the real vaccine.
Thanks for an insightful detail on almost all vaccines till date. Was keen to know more about the Indian Covaxin Vaccine and Sputnik V if possible.
Thanks & Regards
Excellent information.. Some more questions though:
1) has anyone done a study showing efficacy if the second dose was different vaccine?
2) what if the efficacy of each vaccine 2 week after the first vaccine, after a month etc. then after the second dose?
3) now for the really tricky question. Just over 1 million people have been infected and recovered. But how many more have been exposed and are asymptomatic or had mild symptoms and never even got tested. If we tested everyone and many wer already immune naturally could we make the limited vaccines go further /faster?
Government should only advise Pfizer,Moderna and Sputnic because these are more than 90% effective, in Pharmaceutical Science any product or raw material the potency or efficacy of which is less than 90% like Astra Zeneca is considered failed and unacceptable.
Astra Zeneca and other low potency vaccines are not only failed vaccines but also dangerous because 7 people have already died.
Not quite accurate.
Phizer, Moderna and Sputnik were developed and tested in trials before large numbers of varriants began to spread. It is now suspected their efficacy will begin to slip once the varriants come into play.
Near the beginning of this paper, it was clearly stated in large, bold letters “We should be cautious in comparing these trials since they used different ways to measure efficacy, says Alison Thompson, associate professor at University of Toronto.”
Read the 7th paragraph.
They were not tested against the same disease. Pfizer and Moderna tests were completed before the emergence of the mutant strains. Your advice might have to be turned on its head. Pfizer and Moderna have not shown 50% efficacy against the variants that are now dominant.
Government should only advise Pfizer,Moderna and Sputnic because these are more than 90% effective, in Pharmaceutical Science and products any thing less than 90% like Astra Zeneca is considered not acceptable and failed.
Astra Zeneca and other low potency vaccines are not only failed vaccines but also dangerous because 7 people havew already died.
M.AKRAM NIAZI
I would like to know which vaccine is the best for me? I have epilepsy asthma and allergies I’m 35 years old and have 2 young kids one will be 4 years old May and the other is 2 years old my kids are 18 months apart so I don’t want to get them sick or end up in the hospital or something else happening due to an adverse affect from a vaccine my husband is an assential worker.
I can understand your worries, and any illness is disruptive. All the scientific talk can easily get overwhelming. Overall, the evidence seems crystal clear though: get the first vaccine you can get. They are all very good. They are all safe. NO vaccine ever offers bullet-proof disease prevention!! They are all very good though. Dig deep enough (and it helps to have a science background) and the differences between the vaccines are pretty irrelevant to ordinary people. It is only academics and policy makers who need to explore the details. All the best!
According to The Lancer (May 6, 2021), AstraZeneca is 76% effective between 22 and 90 days. Why are you quoting 62%?
As with any study, the sample size will determine the resolution of the results. Some margin of error is expected.
Just like the error in your dates, one should not expect exact, perfection everywhere.
Very, very interesting article. Congratulations. Will there already be some comparative studies (similar to this one) that include the other two vaccines that are being applied in various countries: CoronaVac and Sputnik? Thanks.
I think it should be noted that the science that we are trying to follow is developing as we speak, live, and make decisions and revisions to the decisions. Our leadership is working with the hand that has been dealt to them.
Sometimes it looks as if the policy makers don’t know what they are talking about because their recommendations change over time. That is the nature of science as it develops. There are uncertainties, improvements, and he need to revise conclusions. Science is iterative. We are fortunate to be able to benefit from it in a timely manner. Many in the world have no access currently to vaccines. Nor do the government leaders in some countries really believe that there is a problem and thereby inhibit effective action. I am particularly impressed that in BC, the appointed expert in public health, Dr. Bonnie Henry, is even handed, calm, and as reassuring as she can be. Her experience in other notable pandemic situations has seasoned her responses to the crisis, and we are the beneficiaries.
I would sincerely like to know that too. What is the point of having a first shot and then leaving the patient
vulnerable for 4 months? (especially when the recommendation is 3-4 weeks. I thought we were ‘following
the science’?
The reason for the two-shot schedule IS the science. That is the method that creates in the end the strongest degree of protection. Giving the same overall dosage in one shot just doesn’t work as well. It does, however, work to some degree, so the patients are not left with NO protection; they are just primed to have an enhanced reaction after a second shot.
The departure from that, to a longer interval between shots, is to stretch the supplies of vaccine so that more people can have at least some protection. Suppose we give the first shot to one million people. We have given them some protection, and of particular importance, good protection against severe and lethal cases. If we give their second shot to people for whom we have no vaccine, and the first group wait until production and delivery catch up, we will give coverage against the worst cases to twice as many people, instead of enhanced protection to some and no protection to others.
Website is very helpful. I apreciate it !
Considering that now Canada (Ontario) has 4 month delay for the 2nd dose of vaccine (Pfizer) what is the efficiency or protection if you have only first dose taken ?
Thank you
Thank you, Catharines. It was great to have such an unbiased perspective. We’re up for the Pfizer vaccine in 10 days I believe, since we will be going to an established mass vaccination site in Hamilton. I was wondering though, can we expect milder reactions as we regularly get flu vaccines and have had the shingles vaccine among many others?
Hi Catherine,
Why did the FDA recently rate the AstraZeneca at a 76% efficacy rate while Health Canada continues to present a 62% efficacy? Which rate is it?
Second question, why have we not seen refreshed data on Moderna and Pfizer actual efficacy rate with 100,000,000 US citizens vaccinated?
Yes taking any of the vaccines is an improvement but there still seems to be a significant performance gap between AstraZeneca and the Moderna/Pfizer alternatives.
I have seen a comparative document several weeks ago that included preservative in multi dose vials of the approved covid vaccines. In Canada, we seem to only have received multidose vials and these were listed as having thimerosol in them. The J&J Janssen was listed as single dose only and doesn’t have it. My pharmacist suggested this is true. Now I can’t find that type of comparative information between the vaccines.
have there been been incidents of blood clogs related with any vaccine? if yes, perhaps that was worth reporting.
If you research stats on women’s risk for developing blood clots due to birth control – you will find it is astronomically higher than any Covid vaccine. That has always been worth reporting, yet only now that clots are a possible symptom for men or senior populations it is a concern. Those senior women have been at risk of clotting if they’ve been on any hormonal form of contraception in their lives well before these vaccines. The risk for clotting or strokes is a risk in many medicines, vaccines and especially birth control methods at much higher rates.
what about the study i read, which states that astra zeneca is only 10.4% effective against the south african variant, which is sweeping canada, at the present? justin trudeau does not inspire confidence, even after giving canadians his personal guarantee, that astra zeneca is safe. anyone who takes medical advise, from a failed drama teacher, deserves the consequences. i won’t be lining up, for that vaccine, and, good luck to those who will.
Good question. The 10.4% number is specifically for mild symptoms. It is important to first understand that vaccines do not make people bullet-proof (as much as the public likes to believe that), it is simply untrue. They do make your body able to fight off the disease far faster. Sometimes, fast enough to prevent you from ever showing any indication of the disease. Sometimes you still show hints of it before you recover. That’s how vaccines are. Can *any* Covid vaccines fend off the variants fast enough to prevent even a hint of the disease?? Or at least fast enough to reduce/prevent infecting other people?? And if so, how often?? NO ONE KNOWS YET. We know even less about the latest variants. All we know is that it looks like they reduce (not stop) the spread of the original virus, and the UK variant (Astazeneca appears to be significantly curbing the spread in the UK) but exact numbers simply are not available yet. So bottom line: The 10.4% stat just tells us existing vaccines may keep us a lot healthier, but certaibly variants are still going to spread aggressively and give us mild symptoms. The number is not a good sign for ANY existing vaccine, but not horrible either because the vaccines still keep us from getting any sicker than a bad cold. Maybe future vaccines will do better at stamping out the variants and we won’t even have mild symptoms from Covid in a couple years or so. We will see. I hope that helps.
We are Ontario seniors. My spouse received the first dose of Pfizer vaccine yesterday and has only had some discomfort at the injection site. With Ontario’s recent announcement, that AstraZeneca vaccinations will be available through more pharmacies, I have registered at my local pharmacy and am trying to educate myself about the benefits of one vaccine over another. The recommendation to take any available vaccine is widespread but I want to be as well-protected as I can be. Looking at the first graph, above, it would appear preferable to wait for either Pfizer or moderna but, reading the comments from Alison Thompson regarding efficacy standards, I am unsure. I do have a concern that, because of the new age restrictions around AstraZeneca, it may become the only option at mass vaccination clinics so the others can be saved for the under 55 population. If that becomes the reality, waiting for Pfizer or moderna is pointless.
The UK has achieved truly dramatic reductions in covid infections, primarily through the use of the AstraZeneca vaccine. It is clearly effective in ‘the real world’. As to blood clots – this is a ‘one in a million’ event. Over four million people in the UK have had covid, over 100,000 have died of covid, and under 20 have had blood clots following vaccination. I would accept AstraZeneca in a flash if it were offered to me.
Yes, but what if you are the one to die?! It’s kind of like playing Russian roulette.
This reply is actually to Lee-Anne but it doesn’t have the reply button under her; 4 in 100,000 people got blood clots after Astra Zeneca (which you could expect in the general population anyway but I digress, whereas 16,500 in 100,000 people got blood clots after having covid. So, if you remain unvaccinated then you are TRULY playing russian routlette as you said, as the rate of blood clots after actual infection is over 4000x higher.
Elaine – My boyfriends parents and my father received the 1st dose of AstraZeneca weeks ago & had slight discomfort in the injection site but no other symptoms otherwise. The stats of efficacy per each different vaccine are skewed as this article brings up. As for my own research & what is available to me, I have an appointment to receive the Pfizer. There are risks with each but I agree with your concern of which vaccine will be available when. I know there are also multiple Shopper’s Drug Marts that are doing 24/7 vaccinations – overnight walk in’s welcomed. Good luck!
LeeAnne – Have you ever taken hormonal birth control? You’ve already experienced playing Russian Roulette with your chance of stroke and blood clots if so. 3-9 / 10 000 women will develop blood clots due to birth control – thats one whole 0 knocked off of the population #’s for the vaccine stats…
Would you consider making this excellent and helpful synopsis available as a pdf to download?
Hit print on your computer. Look under the FILE to get the Print command. And Look for the “save as a PDF” command.
All the best!
Thank you. Be blessed.
Why is Canada waiting so long for vaccines?? While the U.S. is so far ahead, even with their 10 x the population?? We did put in pre-orders for the strongest, & first couple of vaccines, at the same time, wih European sippliers, did we not?????
No, Canada did NOT put in orders at the same time as other countries. The federal government chose to wait and see which one would be effective and needed to complete our own review. For comparison, Israel was the first country to order vaccines in June 2020 and they have vaccinated >60% – the highest in the world. Waiting has cost Canadian lives.
Good job!
Spot on report on the difference of all COVID vaccinations. I received the Moderna vaccination and felt a bit under the weather so it’s nice to see that it’s normal. Too bad I had to go through a Canadian site instead of my native USA site to get the information. Thank you
Great work! It’s good to find the stats in one place. A comment, though, regarding the AstraZeneca vaccine; it appears that the numbers you are using are for doses closer to the 12 week delay that is now recommended. The Lancet states that the efficacy for a delay of 6 weeks or less between doses is 55.1% but with 12 weeks or over, is 81.3%. Another study cited 54.9% and 82.4%. Note that the efficacy of the AstraZeneca vaccine was never tested on the 56 + age cohort and the vaccine has not received approval in the U.S. My vaccine appointment is tomorrow and I am seriously thinking of refusing it should it be AstraZeneca as I don’t believe it should be offered to a cohort who was never studied. Isn’t that why there are no plans to vaccinate children at this time, for lack of studies on that age cohort? The constant vaccinating positions of political and medical spokespersons, in this context, does not inspire confidence; surely this is a political decision.
‘vaccilating’ not ‘vaccinating’ lol, bloody autocorrector!
I believe that Our Prime Minister has dropped the ball and let us down! Even though the Prime Minister of Canada was aware of a pending pandemic early last year, our Government leaders have fallen far l behind the United States in acquiring and administering an adequate supply of proven vacines. To make matters worse, Health Canada with our Government support has just approved the least effective vaccine for our most vulnerable seniors age 65 and Older. It is outrageous that the Government thus far has not allowed Seniors to make their own decision as to the vaccine that they wish to have. The apparent decision appears to be due to a lack of supply of Pfyser and Moderna vaccines. Whose fault is that?. I believe that the AstraZeneca approval for Seniors is financially and politically motivated. It looks like an election will be in the cards this fall. PLEASE VOTE! Don’t lose sight of who is looking after your best interests!
I agree. Our Prime minister has done a a pathetic dismal job of acquiring vaccines Talked a lot of how many millions of doses were being shipped but I doubt he ever had clear cut confirmations on these contracts. 35 – 40 in the. world How was this allowed to happen ?
Why is Quebec and the rest of Canada vaccinating people who are 65 years and older withe the AstraZeneca vaccine if it is not recommended for that age group?
Nice review but let’s temper our jubilation. My annual flu shot is just that – a flu SHOT – to stimulate my immune system to generate antibodies to fight off a virus or two — hopefully, as we know picking the star virus for the next season is always a bit of a gamble.
These shots by Pfizer, AZ, J&J, Jansen et al are just that – covid SHOTS – which should merely be likened to a treatment – not the truest definition of a vaccine. Smallpox, MMR, diphtheria – these are vaccines – get them and you don’t get the illness brought on by those bacteria or viruses.
If you get the shot you can still get covid 19, you can probably transmit covid-19 if you get covid-19 and you can still die from covid-19 if you get covid-19. However the likelihood is much, much reduced – and this is a very good thing especially for our senior 65+ folks. If you are in the 20-60y age bracket, according to the CDC, you already have a 99.95% chance of getting over covid if you get coivd – those are some real good odds. This is why Canadian public heath agencies need to get cracking and jabbing our seniors as opposed to announcing that they are soon going to get cracking and jabbing our seniors.
Our Prime Minister has let Canadians down but not securing ample covid shot supply now – the US has jabbed 65 million already. Our public health agencies have let us down as there are very few covid shot campaigns running presently for our at home seniors – why is this, why are the shots still in the fridges and not in arms? These are the real questions that need to be asked…and answered.
Because I am severely anaphylaxis with many allergies should I wait for the Jansen vaccine. I’m 63.
This is the clearest and most comprehensive explanation of the vaccines and their side effects I have come across. Thank you for sharing this work. The graphics are so helpful.
Wow! I am going to share this article with my Family Med colleagues! We are all struggling a bit to answer these questions your colourful and concise article provides easy to understand and explain answers! Thank you !
Thanks for the information
This is an exceptionally clear and informative article. I love the thoughtful infographics. Must read, must share!