Editor’s note: This is an evolving story. As of May 11, both Alberta and Ontario have paused the use of the AstraZeneca vaccine. Click here for our most recent coverage of the vaccine.
Ontario and Alberta are offering the AstraZeneca-Oxford COVID-19 vaccine to people 40 years of age and older starting today.
As pharmacies and family doctor offices gear up to give this two-dose vaccine to more and more Canadians, here are five things we all need to understand about it.
1. This vaccine is very effective at preventing severe illness, hospitalization and death due to COVID-19
Data from clinical trials on AstraZeneca show that the vaccine is nearly 100 per cent efficacious in preventing severe illness, hospitalizations and deaths. Real-world data from the U.K. – where the majority of citizens received AstraZeneca – confirm the drastic decline in hospitalizations and deaths due to COVID-19 post-vaccination, where the dominant strain is B117. This data is extremely reassuring to us here because the B117 is currently the dominant variant in Ontario.
2. There is a very small, but real, increased risk of side effects
The AstraZeneca vaccine has been associated with rare but serious blood clots accompanied with thrombocytopenia (low platelets). This has been termed Vaccine-induced Immune Thrombotic Thrombocytopenia or VITT. The European Medicines Agency observed six cases in 6.8 million doses, for an event rate of under one per million doses. In the Canadian context, we have seen two cases after more than 500,000 doses of the vaccine. The absolute risk for these events remains low overall.
Individual risk is not the same as absolute risk and can be higher or lower depending on age or gender, among other factors. For example, the risk of VITT might be higher than one per million in women under 30. Globally, the majority of these events occurred in women in the 20 to 55 age group and typically within four to 20 days post-vaccination. VITT is treatable if detected early.
3. The benefit of AstraZeneca substantially outweighs the risk during this third wave
Nearly everything in life is a balancing act between risks and benefits. But we are more likely to take calculated and informed known risks than new ones. For example, we hop into our cars every morning to drive to work despite the fact that fatalities due to motor vehicle accidents number 50 in a million annually (and that doesn’t include injuries).
In Ontario, we are on the brink of a health-system collapse. Our ICUs are admitting younger and younger patients. Field hospitals are being set up and pediatric ICUs converted to treat adults. Given the degree of community spread of COVID-19 variants in Ontario, there is a real risk of getting a variant that must be considered in any risk-benefit calculation around vaccines. As well, the vaccine not only provides individuals with protection but also indirectly protects those around them.
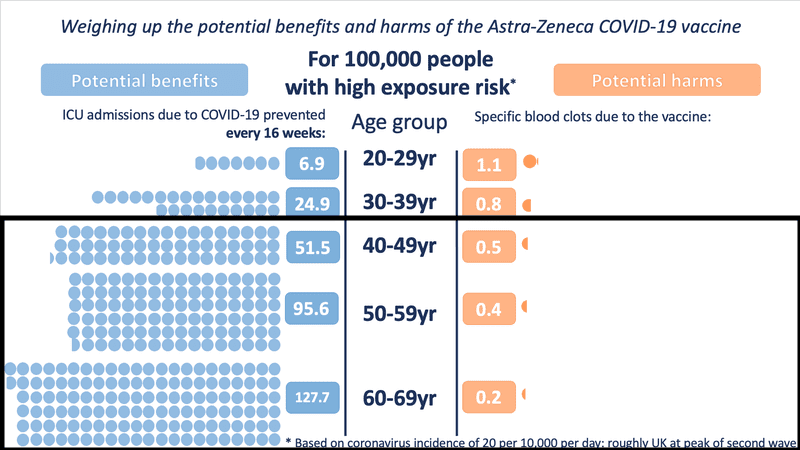
The Winton Centre for Risk and Evidence Communication modelled UK data to illustrate the differences between the benefits offered by the vaccine and small risks of adverse effects. In every situation, including those with low COVID-19 exposure risks, the benefits of AstraZeneca outweigh the risks in those over the age of 30 because the risk of severe COVID-19 infection increases with age.
In Ontario, we are approaching an incidence similar to the high-risk modelling shown in the chart, where the benefit for those over 40 is substantially higher than the risk of side effects:
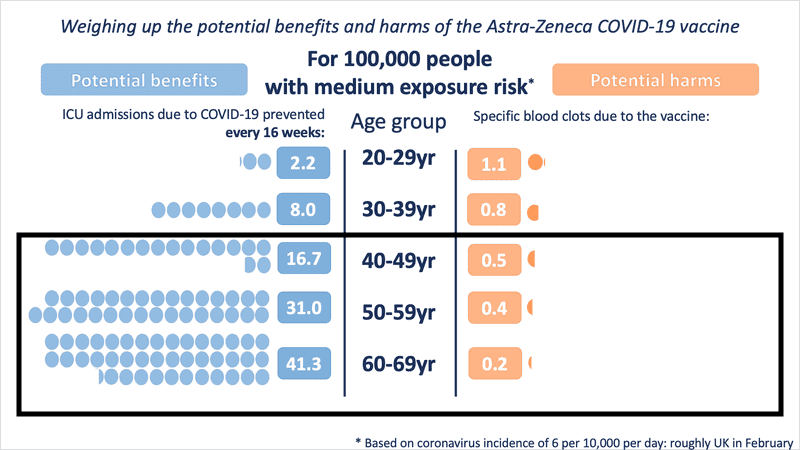
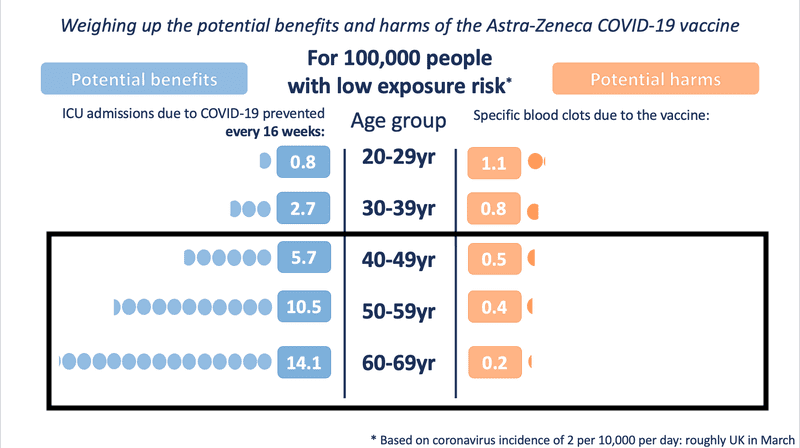
Even in medium- and low-exposure risk scenario modelling, the benefit is substantial as age increases. However, for those under the age of 30, the risk-benefit changes in a low COVID exposure situation.
4. The pause in use does not mean the vaccine is unsafe
The National Advisory Committee on Immunization (NACI) recommendation to pause use of the AstraZeneca vaccine on March 29 was not a confirmation it is unsafe or that approvals were rushed. It is almost impossible to identify such rare side effects in a clinical trial setting. It is only with real-world use of a vaccine in millions of people that you can identify rare reactions.
The brief pause in use for people under 55 is due diligence, done to take all adverse events seriously and ensure COVID-19 vaccine safety.
Pausing use allowed us to better understand real-world data, including incidence and risk of these rare clots. It also served to increase health-care provider and public awareness, ensuring complications can be swiftly recognized and treated appropriately if they occur.
5. Bottom line? Take the first vaccine that is offered to you.
Demand for vaccines is high going into the third wave, and rightly so. But the real-time reporting around the AstraZeneca vaccine and its rare side effects has been confusing for the public. It’s important to not only address safety concerns with empathy and understanding, but also to communicate that due diligence is being done on all vaccines authorized for use in Canada.
The AstraZeneca vaccine is highly effective in preventing severe illness, hospitalization and death due to COVID-19. While it is not risk free (few things in life are), it is safe. And the risk of becoming severely ill with a COVID-19 variant is real. Our advice is to take the first vaccine that is available to you.



The comments section is closed.
I got AstraZeneca for both vaccines I have had so far. The other vaccines are indicating a third vaccine is necessary due to the new variants. Should I get another vaccine after being double vaccinated with AstraZeneca?
None of them are safer. They all make the spike protein which causes clotting.
This seems to be clear misinformation.
1) There are dozens of serious side effects aside from blood clots. Why ignore all serious side effects apart from blood clots?
2) The numbers above don’t have a clear source so can’t be checked. For example, if you look at the UK’s Yellow card data which can be looked up here https://yellowcard.ukcolumn.org/yellow-card-reports#%5Bobject%20Object%5D and considering the population and percentage vaccinated the rate of reported adverse events is around 0.7% of vaccinated people (doing a rough ballpark calculation) and we know that most events aren’t reported. That’s 7 per thousand and 700 per 100,000, a rate that dwarfs the alleged “benefits”. The VAERs data in the US likewise shows a safety profile worse than any other vaccine in history with more deaths for the covid vaccine than all other vaccines combined for decades.
The Yellow card system gives a death rate of around 4 per 100,000 vaccinated, and this doesn’t include long-term deaths caused by e.g. heart damage or auto-immune disorder.
The ICU admissions for covid aren’t necessary in a world where ivermectin, hydroxychloroquine triple therapy, vitamin D supplements, IV vitamin C etc exist.
Imagine trying to force an experimental treatment on people when real and effective conventional treatments exist. This is madness.
Just a few concerns. How come you don’t discuss the antibody-dependent enhancement effect? I know that coronavirus vaccines in the past have had a really bad track record of this. Also, is it not unfair to calculate risks only on the known side-effects? As far as I can tell, this vaccine and the others are still technically “experimental” as in not fully approved. Obviously there cannot be long term data yet – and some things take months or years to show up. How can I know it is still the best option when the risk for those under 50 is so small?
Exactly. And the data that’s already been collected and is misrepresented here shows a terrible safety profile. Around 6000 dead in the US already. 1400 in the UK.
I expect this comment will be deleted as this seems pretty biased.
What if you know your family history of blood clots, is a history of drug induced blood clots? Passed down from your grandmother to father, you may have a 50/50 chance of producing the antibodies in drug-induced immune thrombocytopenia? Would you still roll the dice and get AstraZeneca? No medical professionals I’ve spoken to have been able to advise me.
Hi There,
I have received my first Astrazeneca from Shoppers March 19th. Is the second shot is going to be available soon? I would also like to know if the second shot is stronger than the first one or is it just the same shot. My spouse received Pfizer and has his date for his second shot and it would be nice if I could have a date as well.
Thank you.
Hi, Good article. The analysis misses the point however. The AZ vaccine is effective, all of the vaccines will probably have close to the same real world effectiveness when all is said and done. Sure it is worth the risk to take AZ to prevent COVID. The real question is however, why should someone take AZ when Pfizer and Moderna are safer. The only reason to do so is that you can be protected earlier if offered AZ before the others. Someone should do the risk calculation to show that you should wait no more than “x” days to receive a mRNA vaccine, over AZ. After that time it is worth taking the additional AZ risk for the extra time you are protected. Cheers, Walt
Thank you for the info.Very useful.
I really do not understand the delay (besides the fact we do not have any) in receiving our 2nd vaccine the SCIENCE stated a time line of 14-28 days I received Pfizer on April 3 , 2021 – Second date – July 26,2021 . This goes against “all logic/science” . Trudeau’s Government failed to ensure delivery of these vaccines because they did not Contribute to the development of them at the get go therefore, they are now begging for it. NO $$$ NO PRODUCT !!
What guarantee do we have from our HEALTH EXPERTS by prolonging our Second shots that this will not be harmful to our ongoing health ?? This is what happens when “Politicians” get involved with a Health crisis ..nothing but a a mess !! Thought they would of learned something from TRUMP’s playbook ..NOPE !
If I’ve already had the first dose of the Pfizer vaccine – 14 days ago. Is there any harm in receiving a first doze of the AZ vaccine?
Please do not do this for 3 reasons.
1) You are still generating an immune response from the Pfizer vaccine and that probably peaks around the 2-4 week mark so you’re not “done” with your current vaccine.
2) We don’t know how the Pfizer + AZ will interact. They are looking into it. But we just don’t know. It could be a tasty and beneficial combination (like peanut butter and jelly) or it may end up causing a problem (like vinegar and chocolate). It’s best for the chef (in this case, the trials) to test it out rather than yourself.
3) For both equity and safety reasons. EVERY dose is tracked and the vaccinators know exactly what you’ve had. At least in Ontario
Excellent